
* Autor de contacto:
zamora.martin@inta.gob.ar
Recibido:
09-06-23
Recibido con revisiones: 27-07-23
Aceptado:
03-08-23

CROP DIVERSITY IMPROVES CARBON, NITROGEN AND SOIL BIOLOGICAL
FUNCTIONS IN AN AGROECOLOGICAL SYSTEM
Jimena Ortiz 1, Valeria Faggioli 1, Martin Zamora 1 *, Monica Boccolini 1,
Claudio Lorenzon 1, Vanesa Pegoraro 1, Luciano Gabbarini 2
1 Instituto Nacional de Tecnología Agropecuaria, Argentina.
2 Universidad Naciona de Hurlingham, Argentina.
ABSTRACT
Agroecological management is emerging as a promising alternative to current agricultural management, which is associated with deterioration of environmental quality and soil fertility. Therefore, the aim of this study was to evaluate the conversion from conventional to agroecological management by analysing soil chemical and microbiological properties. This study was carried out in the Barrow Experimental Farm of the National Institute of Agricultural Technology (INTA), Buenos Aires, Argentina, where two treatments were evaluated: agroecological (AE) and conventional (CV) management. Samples were taken at 0 - 10 cm depth, and several soil chemical and microbiological parameters were determined. The AE management resulted in an apparent restoration of soil fertility, with increases in soil organic carbon (SOC), total nitrogen (TN) and pH of 21%, 16% and 3%, respectively. AE management also led to an increase in the activities of enzymes involved in the carbon cycle: cellobiohydrolase (CBH) and β-glucosidase (BG), nitrogen cycle: N-acetyl-b-glucosamine (NAG) and sulfur cycle: arylsulfatase (SUL), as well as an increase in the microbial biomass carbon and in the diversity and richness of the bacterial community (p<0.05). Bacterial and fungal communities differed between treatments (PERMANOVA, bacteria p<0.017 r2=0.1074; fungi p<0.001, r2=0.1973). The bacterial and fungal communities of the AE management were the only ones that correlated positively and significantly with the measured properties, confirming their key role in this system. The bacterial community correlated with the parameters SOC, TN, BG and SUL, while the fungal community correlated with SOC and BG. These results confirm the importance of improving above and belowground biodiversity to maintain or restore soil fertility.
Keywords: Microbial biomass, Enzymatic activity, Microbial diversity, Agroecology.
DIVERSIDAD DE CULTIVOS MEJORA EL CARBONO, nitrogeno Y LAS
FUNCIONES BIOLÓGICAS DEL SUELO EN UN SISTEMA AGROECOLOGICO
RESUMEN
El manejo agroecológico está surgiendo como una alternativa prometedora al manejo agrícola actual, que se asocia con el deterioro de la calidad ambiental y la fertilidad del suelo. Por lo tanto, el objetivo de este estudio fue evaluar la conversión del manejo convencional al manejo agroecológico mediante el análisis de las propiedades químicas y microbiológicas del suelo. Este estudio se llevó a cabo en un campo de la Chacra Experimental Barrow del Instituto Nacional de Tecnología Agropecuaria (INTA) en Buenos Aires, Argentina, donde se evaluaron dos tratamientos: manejo agroecológico (AE) y convencional (CV). Se tomaron muestras de suelo de 0 a 10 cm de profundidad y se determinaron diversos parámetros químicos y microbiológicos del suelo. El manejo AE resultó en una aparente restauración de la fertilidad del suelo, con aumentos en el carbono orgánico del suelo (COS), nitrógeno total (NT) y pH del 21%, 16% y 3%, respectivamente. El manejo AE también condujo a un aumento en las actividades de enzimas involucradas en el ciclo del carbono: celobiohidrolasa (CBH) y β-glucosidasa (BG), ciclo del nitrógeno: N-acetil-b-glucosamina (NAG) y ciclo del azufre: arilsulfatasa (SUL), así como un aumento en el carbono de la biomasa microbiana, y en la diversidad y riqueza de la comunidad bacteriana (p<0,05). Las comunidades bacterianas y fúngicas difirieron entre los tratamientos (PERMANOVA, bacterias p<0,017 r2=0,1074; hongos p<0,001, r2=0,1973). Las comunidades bacterianas y fúngicas del manejo AE fueron las únicas que se correlacionaron positiva y significativamente con las propiedades medidas, confirmando su papel clave en estos sistemas. La comunidad bacteriana correlaciono con los parametros COS, NT, BG y SUL, mientras que la comunidad fungica correlacionó con COS y BG. Estos resultados reafirman la importancia de mejorar la biodiversidad aérea como subsuperficial para mantener o restaurar la fertilidad del suelo.
Palabras clave: biomasa microbiana, actividad enzimática, diversidad microbiana, agroecología.
INTRODUCTION
Agriculture is one of the largest productive sectors in the world. Pasture and cropland occupy around 40% of the habitable land on Earth and provide habitat and food for numerous species (Food and Agriculture Organization Statistics [FAOSTAT], 2020). Although in Argentina, as in many other Latin American countries, 90% of agricultural lands are under no-tillage practices (Albertengo et al., 2013), cropping practices are based on the use of genetically modified crops, poor crop rotation, high dependence on chemical pesticides (Altieri and Nicholls, 2017), and over-fertilization or under-fertilization (Novelli et al., 2023). Consequently, agriculture is associated with the impoverishment of environmental quality and a remarkable decline in soil fertility (Sainz Rosas et al., 2019). To ensure long-term food security while preserving natural resources, sustainable agricultural practices should be adopted. In this context, agroecological management emerges as a promising alternative, as it is conceived as a systemic approach that includes productive, environmental, and social components (Wezel et al., 2014).
Gliessman (2012, p. 366) defined agroecology as the use of ecological principles and concepts for the design and management of sustainable agroecosystems. The basic principles of agroecology are diversity, efficiency, recycling, regulation, and synergy. Multi-species crop planting, crop rotation, cover crops, green manures, and reduced tillage are the most widely adopted practices to improve the ecological functioning of systems (Wezel et al., 2014). Strategically managed plant diversity increases crop yield stability, pollinators abundance and diversity, and weed and pest suppression (Isbell et al., 2017). Despite the growing evidence of the aboveground benefits of agroecological management, the belowground effects remain poorly understood.
Soil is one of the most biodiverse habitats on Earth. It is estimated that 1 g of soil contains more than a billion bacterial species comprising tens of thousands of different taxa, more than 200 m of fungal hyphae, and a wide variety of nematodes, worms, and arthropods (Bardgett and Putten, 2014). Soil microorganisms play a crucial role in many ecosystem services, such as soil formation, nutrient cycling, and plant health (Bardgett and Putten, 2014). However, they are not static and can be altered by agricultural practices and environmental gradients (García-Orenes et al., 2013). Under neglected soil management, sensitive microbial species tend to disappear, while dominant species may predominate, with negative consequences for agroecosystems functioning (García-Orenes et al., 2013). Therefore, the identification of agricultural practices that alter the composition and function of microbial communities is essential not only to the pursuit of sustainability, but also for the conservation of the soil microbial biodiversity.
Key processes, such as carbon turnover and nutrient cycling mediated by enzymatic reactions, take place in the soil. The bioavailability of nutrients is catalysed by enzymes secreted by soil-dwelling organisms, such as bacteria, fungi, plants, and animals, as well as enzymes stored in humus and mineral complexes (Dick, 1994). The amount of enzymatic activity that, occurs under specific biotic and abiotic stress conditions, provides information about the ability of soils to perform biogeochemical reactions (Nannipieri et al., 2018). As enzymatic activity is sensitive to the type of soil management, it can be used to monitor changes in soil quality and health (Dick, 1994). In cultivated systems, the enzymatic activity is higher when cover crops are present in the rotation (Bowles et al., 2014; Brennan and Acosta-Martinez, 2019). In addition, the type of the cover crop produces different responses on the soil enzyme activities, with legumes being associated with higher enzyme levels than grasses (Duchene et al., 2017). However, other authors have shown that cover crops type does not affect soil enzyme activity (Brennan and Acosta-Martinez, 2019). The long-term use of organic amendments is also a positive practice to increase overall enzymatic activities as compared mineral fertilization (Brennan and Acosta-Martinez, 2019; Mäder et al., 2002). Among the possible alternatives for soil management, crop diversification is one of the greatest stimulators of soil enzymatic reactions (Isbell et al., 2017). The presence of roots of different plant species provides a variety of root exudates and plant debris, which constitute a variety of habitats for soil life and recycling processes (Gessner et al., 2010; Lange et al., 2015).
Compared to conventional agriculture, agroecological management can promote soil biological functioning mainly by reducing or avoiding external mineral fertilizers inputs and increasing crop diversity. However, whether this is true after conversion from conventional to agroecological management remains poorly understood, particularly when conventional agriculture is carried out under sustainable practices of crop rotation and no-tillage. The aim of this study was to evaluate the impact of the conversion from conventional to agroecological management by analysing soil chemical and microbiological properties six years after implementation. To study the microbial community composition, we used molecular techniques based on the amplification of bacterial (16S v3) and fungal (ITS1) genes, followed by the analysis of denaturing gradient gel electrophoresis (DGGE). Key enzymes involved in C, N, P and S cycling were evaluated to assess soil biogeochemical functioning. Our hypothesis was that the agroecological practices would have a positive effect on microbial communities and soil biochemical functionality, resulting in improved soil quality and internal nutrient supply.
MATERIALS AND METHODS
Experimental field
The study was carried out in an experimental field located at the Chacra Experimental Barrow, Instituto Nacional de Tecnología Agropecuaria, INTA (-38° 19′, -60° 15′) in the southeastern pampas of Buenos Aires, Argentina. The soils are Mollisol and are classified as fine, illitic, thermic Typic Argiudoll and Petrocalcic Argiudoll (Soil Survey Staff, 2014). The mean annual temperature is 14.8 °C and the frost-free period is from October to March. The area has a humid and subhumid hydric regime. The mean annual precipitation is about 756 mm, concentrated from October to March, and the lowest rainfall occurs between June and August. The experiment started in 2011. The experimental field has 16 ha, divided into two plots of 8 ha each. One of them was managed according to conventional extensive farming practices used by farmers (CV), while in the other plot was managed according to an extensive agroecological farming system (AE) (Fig. 1). The soil-specific zones defined in a previous study were used as field replicates (Aparicio et al., 2018). Apparent soil electrical conductivity (Veris Technologies Inc.,Salina, KS, USA) and elevation (Trimble Navigation Limited, CA, USA) were used as auxiliary information to delimit soil-specific zones within the experimental site (Aparicio et al., 2018).
|
(A) |
(B) |
|
|
|
Figura 1: A. Wheat crop under conventional management (CV). Photograph taken by Martín Zamora. B. Crop diversity (wheat with clover) under agroecological management (AE). Photograph taken by Agustín Barbera.
Figura 1: A. Cultivo de trigo bajo manejo convencional (CV). Fotografia tomada por Martín Zamora. B. Diversidad de cultivos (trigo con trébol) bajo manejo agroecologico (AE). Fotografia tomada por Agustín Barbera.
Cropping management systems
Both the AE and CV management systems combined crop and livestock production under no-tillage (Fig. 2). The AE management focused on the belowground diversity, the equilibrium and cycling of nutrients, as well as the progressive reduction of pesticides use. To achieve these goals, AE management increased the number of species per year (i.e. cover crops and intercrops) and gradually replaced mineral fertilizers with natural sources. At the beginning of the experiment, 100 kg per hectare of di-ammonium phosphate was applied at crop sowing. Then, in October 2013, before planting the summer crop in the third year of the study, minerals were supplied to the cattle. From then on, nutrient replacement was achieved by using legumes to provide N and by providing the cattle P-enriched feed, which recycled the nutrient through faeces when grazing in the field. In the CV system, the crop rotation resembled local farm management (Fig. 2). Nutrient supply was achieved by mineral fertilization with di-ammonium phosphate at doses of 60 to 90 kg per hectare and urea at doses of 18.4 to 200 kg per hectare applied at crop sowing, and by animal faeces. Further descriptions of the management of each plot are detailed in Aparicio et al. (2018), where the external supplies in each system are reported.

Figura 2: Scheme of crop rotation in the agroecological and conventional managements studied during a six-year field experiment. *CDI: Crop diversity index. The index was calculated for each rotation by multiplying the average number of crop species per year by the total number of species across the period studied (Tiemann et al., 2015).
Figura 2: Esquema de rotación de cultivos en los sistemas de manejo agroecológico y convencional estudiados en un experimento de campo de seis años. *CDI: Índice de diversidad de cultivos. El índice fue calculado para cada rotación multiplicando los cultivos por año por el número de especies totales utilizadas en el periodo evaluado (Tiemann et al., 2015).
Soil sampling
In each soil-specific zone for management, the soil was sampled at wheat harvest in November 2016. Five sampling stations for soil-specific zones were established, where composite soil samples (20 subsamples) were collected, at a depth of 0 to 10 cm. Samples were passed through a 2 mm sieve immediately after sampling. Then 200 g of soil were dried at room temperature for chemical determinations, 200 g were stored at 4 ºC until analysis of microbial and enzymatic activities, and 20 g were stored at −20°C for molecular analysis. It is worth noting that the gravimetric water content at sampling was 5–6% w:w, in both treatments, reflecting the previous dry period during the growing season.
Soil chemical properties
Soil organic C (SOC) was determined by wet oxidation, following the Walkley-Black procedure (Black and Walkley, 1934). Total N (TN) was quantified by the semi-micro Kjeldahl method (Bremner, 1996) and extractable P (EP) was quantified according to the Bray-Kurtz method (Bray and Kurtz, 1945). Soil pH was measured at a soil-to-water ratio of 1:2.5.
Microbial biomass and respiration
Microbial respiration was estimated by incubation for seven days at 25 °C according to Alef (1995, p. 214), microbial biomass carbon (MBC) was determined by the fumigation-extraction method (Vance et al., 1987), and the metabolic quotient (qCO2) was obtained as the proportion of C-CO2 in the soil sample released from microbial biomass.
Soil enzyme activities
Microbial activity was estimated by the activity of six enzymes: three involved in the C cycle: cellobiohydrolase (CBH), β-glucosidase (BG), and acetyltransferase (ACE); one involved in the N cycle: chitinase or N-acetyl-b-glucosamine (NAG); one involved in the P cycle: acid phosphatase (AP); and one involved in the S cycle: arylsulphatase (SUL). Measurements were performed with the Omega PolarStar fluorescence reader, using substrates derived from 4-Methylumbelliferone, expressed as nmol h-1 g-1 dry soil (Truong et al., 2019).
Microbial community structure
DNA was extracted from 0.25 g of soil using the MoBio PowerSoil kit, following the manufacturer’s instructions. For the specific amplification of the 16S region (V3), PCR test was performed with the universal primers F341-GC and R534. For specific amplification of the ITS1 region, nested-PCR was performed with the universal primers ITS1/ITS4 and ITS1-GC/ITS2. Amplification products corresponding to each sample were analysed by (DGGE) (Muyzer et al., 1993) consisting of 8% polyacrylamide gels with a linear denaturing gradient of 40 to 60% for bacteria and of 30 to 55% for fungi. Electrophoresis conditions were 60 V for 16 h at 60 °C. The gels were stained with GelRed (Biotium, San Francisco, CA, USA) in 1X TAE for 45 min and visualised under UV light. The DGGE profiles were digitised and analysed with the Gel Compare II software version 6.0 (Applied Maths NV, Sint-Martens-Latem, Belgium). The position and intensity of the bands were used for subsequent statistical analysis and estimation of Shannon-Wiener diversity indices (Shannon 1948) and richness (Menhinick 1964).
Statistical analyses
To evaluate the effect of management on soil chemical and microbiological variables, a Mixed Generalized Linear Model (MGLM) was applied, with management as a fixed effect and areas as random effects. Means were compared using the LSD test at 5% significance. Analyses were performed using the INFOSTAT statistical software (Di Rienzo et al., 2020). The composition of bacterial and fungal communities was analysed using the presence-absence matrix resulting from the DGGE technique. Diversity indexes were compared using MGLM as described above. To evaluate the effect of soil management on microbial communities, permutational ANOVA was used by implementing the adonis function in the vegan package in R (R Development Core Team, 2020). Community composition visualisation plots were analysed using Constrained Analysis of Principal Coordinates and the altgower distance matrix (binary mode) was determined using the labsdv package in R. Linear correlations of chemical parameters, enzyme activities and microbiological variables with the microbial community structure were estimated using the envfit function in the vegan package, and only significantly correlated vectors were plotted (p <0.05).
RESULTS AND DISCUSSION
Soil chemical fertility
After conversion from CV to AE management, soil chemical parameters improved significantly. SOC, TN, and pH were 21%, 16%, and 3% higher in AE than in CV (p<0.05), while soil C:N ratio and EP remained unchanged (Table 1). Increasing SOC levels is important for sustainability and because of the many benefits associated with SOC levels (Doran and Parkin, 1994). Restoring SOC in agricultural soils is a strategy to improve soil fertility because SOC affects many soil properties, including its ability to retain water and nutrients, provide a structure that promotes efficient drainage and aeration, and minimise the loss of topsoil through erosion (Foley et al., 2011). In our study, increased crop diversity in the AE system (Fig. 2) was a key factor to improve SOC (Wang et al., 2017). In previous works (Lange et al., 2015; Zhang et al., 2021), the authors found similar results, i.e. increased crop diversity increases C inputs due to higher rhizodeposition, which could generate more labile C sources for microbial functions, leading to a decrease in the decomposition of the existing soil C.
Table 1: Mean values ± standard errors for soil organic carbon (SOC), total nitrogen (TN), extractable P (EP), pH and C:N ratio, measured in two different management systems: agroecological (AE) and conventional (CV) management. Different letters indicate values that are significantly different (p<0.05).
Tabla 1: Valores medios ± error estandar para carbono orgánico del suelo (COS), nitrógeno total (NT), fósforo extractable (PE), pH y relación C:N, medidos en dos diferentes sistemas de manejo: agroecológico (AE) y convencional (CV). Letras diferentes indican diferencias significativas (p<0.05).
|
Treatments |
SOC |
TN |
EP |
pH |
C:N |
|
|
mg C g-1 |
mg N g-1 |
mg P Kg-1 |
|
|
|
AE |
33.22 ± 0.56 a |
3.00 ± 0.16 a |
22.40 ± 4.82 |
7.03 ± 0.07 a |
11.37 ± 0.57 |
|
CV |
27.78 ± 0.82 b |
2.37 ± 0.08 b |
21.10 ± 2.47 |
6.82 ± 0.10 b |
11.88 ± 0.48 |
|
p value |
<0.0001 |
0.0007 |
0.7608 |
0.0001 |
0.3768 |
Soil pH is not only an important determinant of biogeochemical reactions, but also regulates crucial soil processes (Van Elsas et al., 2019). In agroecosystems, one of the main reasons for soil acidification is N fertilization (Williams, Börjesson and Hedlund, 2013; Ortiz et al., 2020). Restoring soil pH is the best approach to overcome the negative effects of soil deterioration (acidification) and improve soil functions and nutrient availability (Holland et al., 2018). Our results showed that replacing chemical fertilizers with crop rotation and livestock manure could be considered as an alternative to liming on slightly acidic soils.
The implementation of AE relies on the knowledge of the system and on the proper handling of factors such as crop diversity and nutrient recycling, to compensate for the lack of external chemical inputs and maintain productivity. Here we have shown that increasing the number of species in the crop rotation system, combined with intercropping and cover crops of legumes and grasses is a successful practice to improve key soil parameters in a relatively short period after conversion from a conventional system. Nevertheless, it would be necessary to monitor the evolution of non-responding variables, such as EP, before they reach insufficiency levels that could limit crop production (Novelli et al., 2023).
3.2. Soil biogeochemical properties and microbial functions
The AE increased MBC and total enzyme activities (Fig 3). No differences were observed between management systems for MR and qCO2 (Fig 3). Despite the low soil water content at sampling and the high loss of C via MR that would be expected (Harris et al., 1981; Maestre et al., 2015; Manzoni et al., 2012), the observed MR and qCO2 values did not exceed those reported in other studies for degraded soils such as soybean monoculture (Ferreras et al., 2009; Serri et al., 2018). This is important because the soil C storage is related to C use efficiency, which is highly regulated by the rate and efficiency with which soil microorganisms incorporate C into their biomass. This represents the allocation of C to microbial growth versus respiration (Domeignoz-Horta et al., 2020). Several studies have demonstrated an increase in microbial biomass and microbial activity by intercropping legumes and cereals (McDaniel et al., 2014, Song et al., 2007) and by incorporating cover crops (Frasier et al., 2016), especially when they are consociated cover crops (Chavarria et al., 2016), as in this study.

Figura 3: Microbial biomass carbon (MBC), microbial respiration (RB) and metabolic quotient (qCO2), measured in two different management systems: agroecological (AE) and conventional (CV).
Figura 3: Carbono de la biomasa microbiana (CBM), respiración microbiana (RM) y coeficiente metabolico (qCO2, medidos en dos diferentes sistemas de manejo: agroecologico (AE) y convencional (CV).
The AE increased the enzymatic activity, particularly enzymes associated with the C, N, and S cycles: BG, NAG, and SUL (p<0.05). Previous studies have shown the contradictory responses of enzymatic activities. For example, Nannipieri et al. (2018) found that enzymatic activities were strongly influenced by the surrounding environment and C source. However, Wall et al. (2019) reported that enzymatic activities do not always have a linear response to substrate availability and suggested that the factors regulating enzyme production are still unclear. Sinsabaugh et al. (2014) reported that enzyme activities are good indicators of resource demand, as their activities increase with limited resources. On the other hand, Salazar et al. (2011) and Bowles et al. (2014) found higher enzymatic activity at higher soil C and N contents, respectively. Therefore, despite the regulating factors, we found increased enzymatic activities at increased SOC and TN levels in AE.
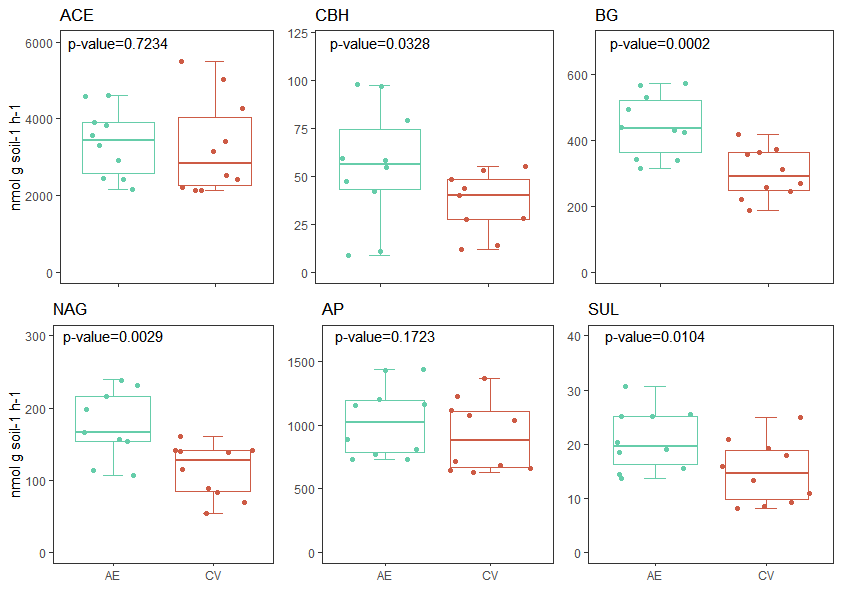
Figura 4: Enzymatic activity of Acetyl esterase (ACE), Cellobiohydrolase (CBH), β-Glucosidase (BG), N- acetyl-b-glucosamine “Chitinase” (NAG), acid phosphate (FA) and sulphatase (SUL), measured in two different management systems: agroecological (AE) and conventional (CV).
Figura 4: Actividad enzimática de acetil esterasa (ACE), Celubiohidrolasa (CBH), N- acetil glusaminasa “Quitinasa” (NAG), fosfatasa ácida (FA) and sulfatasa (SUL), medidas en dos diferentes sistemas de manejo: agroecológico (AE) y convencional (CV).
Cellulose, which is the most abundant component of plant tissues, must be degraded by cellulolytic enzymes, such as CBH and BG, to build soil organic matter and provide a source of nutrients for crops. Higher levels of CBH and BG were observed in AE. The CBH enzyme is present in all fungal cellulolytic cocktails and intervenes in a step prior to the BG in the breakdown of cellulose (Gutiérrez-Rojas et al., 2015). Both enzymes are very important in decomposition, and it has been reported that cellulose has long half-life in neutral pH soils and in the absence of enzymes (Wilson, 2011).
Chitin is the second most abundant polysaccharide in plant tissues and the NAG enzyme is responsible for its hydrolysis (Stoykov et al., 2015). We observed an increase in NAG activity in AE. The C:N enzymatic acquisition ratio was performed to determine the relative limitation of C versus N (Sinsabaugh et al., 2009), and we observed that CV had a higher acquisition ratio than AE (Table 2), indicating that microorganisms in AE are less limited by C, probably due to the greater diversity of crops (Tienman et al., 2015). This may mean that these microorganisms are able to provide more N during periods of high N demand (McDaniel et al., 2014).
The main sources of S in soil are organic compounds, and the release of sulfate is strongly mediated by enzymatic reactions, mainly arylsulphatases (Tabatabai and Bremner 1970). Arylsulphatases are secreted by bacteria and are responsible for the hydrolysis of sulfate esters in soil (Kertesz and Mirleau 2004) in response to S limitation (McGill and Cole 1981). As we found that SUL was higher in AE, we can infer that S supply was highly mediated by enzymatic processes in the AE system. As the nutrient source in AE was the natural cycle of diversified crops and grazing livestock faeces, both practices were strong enough to favourably influence C, N, and S cycling mediated by enzymatic activities. Therefore, our results demonstrate the importance of strategic crop rotation design and nutrient supply for soil functionality.
Table 2: Mean values ± standard errors for enzymatic ratio C:N, N:P and C:P calculated from enzyme activity data measured in two different management systems: agroecological (AE) and conventional (CV). Different letters indicate values that are significantly different (p<0.05).
Tabla 2: Valores medios ± errores estándar para relación enzimática C:N, N:P y C:P calculados a partir de datos de actividad enzimática medidos en dos sistemas de manejo diferentes: agroecológico (AE) y convencional (CV). Letras diferentes indican diferencias significativas (p<0,05).
|
Treatment |
C:N |
N:P |
C:P |
|
|
enzyme ratio |
||
|
AE |
1.57 ± 0.06 b |
0.46 ± 0.03 |
0.71 ± 0.02 |
|
CV |
1.84 ± 0.15 a |
0.43 ± 0.04 |
0.73 ± 0.02 |
|
p-value |
0.0064 |
0.3492 |
0.5060 |
Microbial community structure
Our results demonstrated the sensitivity of microbial communities to increased crop diversity in an agroecological system. Richness and Shannon-Wiener indexes were higher in the AE system than in the CV system for both bacteria and fungi, but with a significant effect (p<0.05) only for bacterial communities (Fig 5). Hartman et al. (2018), who compared a conventional and an organic system, found bacterial communities in root but not in soil samples. They argued that it is more difficult to find an effect on the soil than on the root. In contrast, in the present study, we found effects of the management system on the soil. The response observed in the α-diversity of bacterial communities in the AE can be attributed to an improvement in the stubble quality due to the incorporation of consociated cover crops (e.g., oat with vetch) and intercropping with legumes (e.g., wheat with clover). This is in agreement with Frasier et al. (2016), who reported that after three years of diversifying a sorghum monoculture with legume cover crops, the bacterial community increased and this increase was associated with higher residue quality. Zhou et al. (2011) also reported an increase in bacterial diversity when intercropping was used. Although we found a response of the soil microbial community, further studies should elucidate the composition of the microbial communities inhabiting the roots of crops growing in these contrasting management systems.
Another key factor regulating microbial communities is soil pH. In this regard, we found higher pH in the AE system, and previous studies have reported higher bacterial α-diversity in soils with neutral pH (Fierer, 2017; Rousk et al., 2010). Our results also showed that the α-diversity of fungal communities was less sensitive to soil pH, in agreement with a previous study (Rousk et al., 2010). The higher diversity and richness observed suggest a more resilient and stable system in the face of environmental change due to increased redundancy of activities (Wagg et al., 2014). Greater diversity ensures broad soil processes, such as the C cycle, and even more specific processes carried out by a limited group of microorganisms, such as denitrification and methanogenesis (Ho et al., 2014; Philippot et al., 2013). The community composition (β-diversity) of fungi and bacteria was significantly influenced by the management system. We found a clustering by management practices (Fig. 5). The PERMANOVA confirmed such marked differences between the two microbial communities (bacteria R2=0.1074, P < 0.017; fungi R2=0.1973, P < 0.001). Consistent with the findings of Song et al. (2007), the composition of the fungal and bacterial communities in the AE system was different from that in the CV system. This suggests that the soil communities have different members, which could indicate different community efficiencies and functions (Domeignoz-Horta et al., 2020; Wang et al., 2017). When evaluating the relationships between the microbial community and soil properties (Fig. 5), we observed that only the bacterial and fungal communities of the AE management correlated positively and significantly with the measured properties. The bacterial community in AE was positively and significantly correlated with SOC, TN, and BG and SUL activities, while the fungal community in AE was correlated with the contents of SOC and BG. The results of this study confirm that processes related to organic matter and nutrient cycling in AE are associated with microbial communities’ activities (Lange et al., 2015).
|
(A) (B) |
|
|
|
|
|
(C) (D) |
|
|
|
|
Figura 5: Changes in the bacterial and fungal community between agroecological (AE) and conventional management (CV). A and C: Constrained Analysis of Principal Coordinates (CAP) analysis of the community beta diversity of bacterial and fungal communities based on Altgower distance. The significance of differences between microbial communities was identified using permutational multivariate analysis of variance (PERMANOVA). Vectors represent significant correlations between ordination and soil properties: SOC (soil organic matter), SUL (sulfatase), TN (total N), BG (β-Glucosidase). Ellipses indicate one unit of standard deviation from the centroid of each group. B and D: Community alpha diversity of the bacterial and fungal communities. Microbial abundance was determined based on the presence-absence matrix generated from the 16S and ITS1 rRNA amplicons after DGGE analysis, with significant differences at P < 0.05 and P <0.01, respectively (MLM).
Figura 5: Cambios en la comunidad bacteriana y fúngica entre el manejo agroecológico (AE) y convencional (CV). A y C: análisis de coordenadas principales (CAP) de la diversidad beta de las comunidades bacterianas y fúngicas en función de la distancia Altgower. Las diferencias entre las comunidades microbianas se identificaron mediante el análisis de varianza multivariando permutacional (PERMANOVA). Los vectores representan correlaciones significativas entre la ordenación y las propiedades del suelo: SOC (carbono orgánico del suelo), SUL (sulfatasa), TN (N total), BG (β-glucosidasa). Las elipses indican una unidad de desviación estándar del centroide de cada grupo. B y D: Diversidad alfa de la comunidad bacteriana y fúngica. La abundancia microbiana se determinó en función de la matriz de presencia-ausencia generada a partir de los amplicones de ARNr 16S e ITS1 después del análisis DGGE. Representan diferencias significativas en P < 0,05 y P < 0,01, respectivamente (MLM).
CONCLUSIONS
This study showed the response of microbial communities to the adoption of agroecological management in extensive crop production. Our results suggest that agroecological management is characterised by higher microbial activity and microbial growth than conventional management. These characteristics indicate a more efficient use of carbon in agroecological systems compared to a conventional management system. This shows that sustainable agricultural tools such as increasing crop diversity and the livestock manure can be used effectively to maintain soil quality. Furthermore, these results are directly applicable to particular systems, such as organic and low-input farming, that strive to optimise internal nutrient supply, and can be used to generate data to help farmers consider the adoption or this alternative management, which can produce food while reducing environmental risk and production costs.
ACKNOWLEDGMENTS
The authors thank the Instituto Nacional de Tecnología Agropecuaria (INTA) Argentina. This research was supported by Instituto Nacional de Tecnología Agropecuaria (INTA) through the research projects PNSUELO 1134043 and REDAE 1136021. The authors are deeply grateful to Cristian Cazorla for helping with soil sampling. We further thank the staff of INTA Barrow and INTA Marcos Juárez Research Station, where our research was conducted.
REFERENCES
Albertengo, J., Belloso, C., Giraudo, M.B., Peiretti, R., Permingeat, H., & Wall, L. (2013). Conservation Agriculture in Argentina. In: Conservation agriculture: Global prospects and challenges. (pp. 352-374). Wallingford UK: CABI.
Alef, K. (1995). Soil respiration. In: Alef, K., & Nanninpieri P. (ed.). Methods in Applied Soil Microbiology and Biochemistry. (pp. 214-219). Academic Press. Harcourt Brace and Company publishers, London U.K.
Altieri, M.A. & Nicholls, C.I. (2017). The adaptation and mitigation potential of traditional agriculture in a changing climate. Climatic Change. 140(1), 33-45. https://link.springer.com/article/10.1007/s10584-013-0909-y.
Aparicio, V., Zamora, M., Barbera, A., Castro-Franco, M., Domenech, M., De Gerónimo, E., & Costa, J.L. (2018). Industrial agriculture and agroecological transition systems: A comparative analysis of productivity results, organic matter and glyphosate in soil. Agricultural systems. 167, 103-112. https://doi.org/10.1016/j.agsy.2018.09.005.
Bardgett, R.D. & Van Der Putten, W.H. (2014). Belowground biodiversity and ecosystem functioning. Nature. 515(7528), 505. doi:10.1038/nature13855.
Bray, R. H., & Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil science, 59(1), 39-46.
Bremner, J.M. (1996). Nitrogen-total. Methods of Soil Analysis Part 3-Chemical Methods, (methodsofsoilan3), (Ed.), American Society of Agronomy Madison, Wisconsin, 1085–1121.
Brennan, E.B. & Acosta-Martinez, V. (2019). Cover crops and compost influence soil enzymes during six years of tillage‐intensive, organic vegetable production. Soil Science Society of America Journal. 83.3, 624-637.
Bowles, T.M., Acosta-Martínez, V., Calderón, F. & Jackson, L.E. (2014). Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biology and Biochemistry. 68, 252-262. https://doi.org/10.1016/j.soilbio.2013.10.004.
Chavarría, D. N., Verdenelli, R. A., Serri, D. L., Restovich, S. B., Andriulo, A. E., Meriles, J. M. & Vargas-Gil, S. (2016). Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. European journal of soil biology, 76, 74-82.
Di Rienzo, J., Casanoves, F., Balzarini, M., Gonzalez, L., Tablada, M. & Robledo, C. (2020). InfoStat versión 2020. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Dick, R.P. (1994). Soil Enzyme Activities as Indicators of Soil Quality 1. Doran, JW; DC Coleman; DF Bezdicek & BA Stewart. (eds.). Defining soil quality for a sustainable environment. (pp. 107-124). Madison, Wisconsin, USA.
Domeignoz-Horta, L. A., Pold, G., Liu, X. J. A., Frey, S. D., Melillo, J. M., & DeAngelis, K. M. (2020). Microbial diversity drives carbon use efficiency in a model soil. Nature communications, 11(1), 3684.
Doran, J.W., and Parkin, T. B. (1994). Defining and assessing soil quality. In: Doran, JW; DC Coleman; DF Bezdicek & BA Stewart. (eds.). Defining Soil Quality for a Sustainable Environment. (pp. 3-21). Madison, Wisconsin, USA.
Duchene, O., Vian, J.F., Celette, F. (2017). Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agriculture, Ecosystems and Environment. 240, 148-161. https://doi.org/10.1016/j.agee.2017.02.019.
Food and Agriculture Organization, (2020). FAOSTAT Online Database (Last update Jan, 2023). Available at (https://faostat.fao.org/) (accessed, January 2023).
Ferreras, L., Toresani, S., Bonel, B., Fernández, E., Bacigaluppo, S., Faggioli, V. & Beltrán, C. (2009). Parámetros químicos y biológicos como indicadores de calidad del suelo en diferentes manejos. Ciencia del suelo, 27(1), 103-114.
Fierer, N. (2017). Embracing the unknown: disentangling the complexities of the soil microbiome. Nature Reviews Microbiology, 15(10): 579.
Foley, J.A., Ramankutty, N., Brauman, K.A., Cassidy, E.S., Gerber, J.S., Johnston, M., Mueller, N.D., O’Connell, C., Ray, D.K., West, P,C., Balzer, C., Bennett, E.M., Carpenter, SR., Hill, J., Monfreda, C., Polasky, S., Rockström, J., Sheehan, J., Siebert, S., Tilman, D., & Zaks, D.P.M. (2011). Solutions for a cultivated planet, Nature. 478, 337–342. doi:10.1038/nature10452.
Frasier, I., Noellemeyer, E., Figuerola, E., Erijman, L., Permingeat, H., & Quiroga, A. (2016). High quality residues from cover crops favor changes in microbial community and enhance C and N sequestration. Global ecology and conservation. 6, 242-256. https://doi.org/10.1016/j.gecco.2016.03.009.
García-Orenes, F., Morugán-Coronado, A., Zornoza, R., & Scow, K. (2013). Changes in soil microbial community structure influenced by agricultural management practices in a Mediterranean agro-ecosystem. PloS one. 8, (11). https://doi.org/10.1371/journal.pone.0080522.
GelCompare II. (2005). Vertion 4.602 of Applied Maths NV.
Gessner, M.O., Swan, C.M., Dang, C.K., McKie, B.G., Bardgett, R.D., Wall, D.H., & Hättenschwiler, S. (2010). Diversity meets decomposition. Trends in ecology and evolution. 25(6), 372-380. https://doi.org/10.1016/j.tree.2010.01.010.
Gliessman, S. R. (2012). Quantifying the Agroecological Component Agroecology. In: Researching the Ecological Basis for Sustainable Agriculture. (pp. 366). Springer Science and Business Media.
Gutiérrez-Rojas, I., Moreno-Sarmiento, N., & Montoya, D. (2015). Mecanismos y regulación de la hidrólisis enzimática de celulosa en hongos filamentosos: casos clásicos y nuevos modelos. Revista Iberoamericana de Micología, 32(1), 1-12.
Hartman, K., Van der Heijden, M.G., Wittwer, R.A., Banerjee, S., Walser, J.C., & Schlaeppi, K. (2018). Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome. 6(1), 1-14. DOI 10.1186/s40168-017-0389-9.
Harris, R., Parr, J., Gardner, W., & Elliot, L. (1981). Effect of water potential on microbial growth and activity. In: Water potential relations in soil microbiology. (pp. 23-95). Soil Science Society of America. https://doi.org/10.2136/sssaspecpub9.c2.
Ho, A., De Roy, K., Thas, O., De Neve, J., Hoefman, S., Vandamme, P., ... Boon, N. (2014). The more, the merrier: heterotroph richness stimulates methanotrophic activity. The ISME journal, 8(9), 1945-1948.
Holland, J.E., Bennett, A.E., Newton, A.C., White, P.J., McKenzie, B.M., George, T.S., & Hayes, R.C. (2018). Liming impacts on soils, crops and biodiversity in the UK: a review. Science of the total environment. ٦١٠, 316-332. https://doi.org/10.1016/j.scitotenv.2017.08.020.
Isbell, F., Adler, P.R., Eisenhauer, N., Fornara, D., Kimmel, K., Kremen, C., & Scherer‐Lorenzen, M. (2017). Benefits of increasing plant diversity in sustainable agroecosystems. Journal of Ecology. 105(4), 871-879. https://doi.org/10.1111/1365-2745.12789.
Kertesz, M. A., & Mirleau, P. (2004). The role of soil microbes in plant sulphur nutrition. Journal of experimental botany, 55(404), 1939-1945.
Lange, M., Eisenhauer, N., Sierra, C.A., Bessler, H., Engels, C., Griffiths, R.I., & Steinbeiss, S. (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications. 6, 6707. DOI: 10.1038/ncomms7707.
Mäder, P., Fliessbach, A., Dubois, D., Gunst, L., Fried, P., & Niggli, U. (2002). Soil fertility and biodiversity in organic farming. Science. 296(5573), 1694-1697. DOI: 10.1126/science.1071148.
Maestre, F. T., Delgado-Baquerizo, M., Jeffries, T. C., Eldridge, D. J., Ochoa, V., Gozalo, B., & Singh, B. K. (2015). Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proceedings of the National Academy of Sciences, 112(51), 15684-15689.
Manzoni, S., Taylor, P., Richter, A., Porporato, A.,& Ågren, G. I. (2012). Environmental and stoichiometric controls on microbial carbon‐use efficiency in soils. New Phytologist, 196(1), 79-91.
McDaniel, MD., Tiemann, LK., & Grandy, A.S. (2014). Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta‐analysis. Ecological Applications. 24(3), 560-570. https://doi.org/10.1890/13-0616.1.
McGill, W. B., & Cole, C. V. (1981). Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma, 26(4), 267-286.
Menhinick, E.F. (1964). A Comparison of some Species-Individuals Diversity Indices Applied to Samples of Field Insects. Ecology. 45 (4), 859-861.
Muyzer, G., & Smalla, K. (1998). Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek. 73: 127-141.
Muyzer, G., Waal, E.C., & Uitterlinden, A.G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59, 695–700. https://doi.org/10.1128/aem.59.3.695-700.1993.
Nannipieri, P., Trasar-Cepeda, C., & Dick, R.P. (2018). Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils. 54, 11. DOI 10.1007/s00374-017-1245-6.
Novelli, L. E., Caviglia, O. P., Jobbágy, E. G., & Sadras, V. O. (2023). Diversified crop sequences to reduce soil nitrogen mining in agroecosystems. Agriculture, Ecosystems and Environment, 341, 108208.
Ortiz, J., Faggioli, V. S., Ghio, H., Boccolini, M. F., Ioele, J. P., Tamburrini, P., & Gudelj, V. (2020). Impacto a largo plazo de la fertilización sobre la estructura y funcionalidad de la comunidad microbiana del suelo. Ciencia del suelo, 38(1), 45-55.
Philippot, L., Spor, A., Hénault, C., Bru, D., Bizouard, F., Jones, C. M., ... Maron, P. A. (2013). Loss in microbial diversity affects nitrogen cycling in soil. The ISME journal, 7(8), 1609-1619.
R Development Core Team. (2020). R: A language and environment for statistical computing.
Rousk, J., Bååth, E., Brookes, P.C., Lauber, C.L., Lozupone, C., Caporaso, J.G., & Fierer, N. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME journal. 4(10), 1340-1351. https://doi.org/10.1038/ismej.2010.58.
Sainz Rozas, H., Eyherabide, M., Larrea, G., Martínez Cuesta, M., Angelini, H., Reussi Calvo, N., & Wyngaard, N. (2019). Relevamiento y determinación de propiedades químicas en suelos de aptitud agrícola de la región pampeana. Actas Simposio Fertilidad. 141–158. http://hdl.handle.net/20.500.12123/11824.
Salazar, S., Sánchez, L.E., Alvarez, J., Valverde, A., Galindo, P., Igual, J.M., & Santa-Regina, I. (2011). Correlation among soil enzyme activities under different forest system management practices. Ecological Engineering. 37(8), 1123-1131. https://doi.org/10.1016/j.ecoleng.2011.02.007.
Serri, D. L., Boccolini, M., Oberto, R., Chavarría, D., Bustos, N., Vettorello, C., ... and Vargas Gil, S. (2018). Efecto de la agriculturización sobre la calidad biológica del suelo. Ciencia del suelo. 36(2), 92-104.
Shannon, C.E. (1948). A mathematical theory of communication. Bell System Technical Journal. 27, 379–423.
Sinsabaugh, R. L., Hill, B. H., & Follstad Shah, J. J. (2009). Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature, 462(7274), 795-798.
Soil Survey Staff. (2014). Keys to Soil Taxonomy, 12 th ed. United States Department of Agriculture- Natural Resources Conservation Service, Washington, DC.
Song, Y.N., Zhang, F.S., Marschner, P., Fan, F.L., Gao, H.M., Bao, X.G., & Li, L. (2007). Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biology and Fertility of Soils. 43(5), 565-574. DOI 10.1007/s00374-006-0139-9.
Stoykov, Y. M., Pavlov, A. I.,& Krastanov, A. I. (2015). Chitinase biotechnology: production, purification, and application. Engineering in Life Sciences, 15(1), 30-38.
Tabatabai, M. A., & Bremner, J. M. (1970). Arylsulfatase activity of soils. Soil Science Society of America Journal, 34(2), 225-229.
Tiemann, L.K., Grandy, A.S., Atkinson, E.E., Marin‐Spiotta, E., & McDaniel, M.D. (2015). Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecology letters. 18(8), 761-771. https://doi.org/10.1111/ele.12453.
Truong, C., Gabbarini, L.A., Corrales, A., Mujic, A.B., Escobar, J.M., Moretto, A., & Smith, M.E. (2019). Ectomycorrhizal fungi and soil enzymes exhibit contrasting patterns along elevation gradients in southern Patagonia. New Phytol. 222(4), 1936–1950. https://doi.org/10.1111/nph.15714.
Vance, E.D., Brookes, P.C., & Jenkinson, D.S., (1987). An extraction method for measuring soil microbial biomass C. Soil biology and Biochemistry. 19(6), 703-707.
Van Elsas, J. D., Hartmann, A., Schloter, M., Trevors, J. T., & Jansson, J. K., 2019. The bacteria and archaea in soil. In Modern soil microbiology (pp. 49-64). CRC Press.
Wall, L. G., Gabbarini, L. A., Ferrari, A. E., Frene, J. P., Covelli, J., Reyna, D., & Robledo, N. B. (2019). Changes of paradigms in agriculture soil microbiology and new challenges in microbial ecology. Acta Ecological, 95, 68-73.
Wagg, C., Bender, S.F., Widmer, F., & Van der Heijden, M.G. (2014). Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proceedings of the National Academy of Sciences. 111(14), 5266-5270. https://doi.org/10.1111/nph.15714.
Walkley, A & Black, I.A. (1934). An examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29-38.
Wang, Y., Ji, H., Wang, R., Guo, S., & Gao, C. (2017). Impact of root diversity upon coupling between soil C and N accumulation and bacterial community dynamics and activity: result of a 30 year rotation experiment. Geoderma, 292, 87-95.
Wezel, A., Casagrande, M., Celette, F., Vian, JF., Ferrer, A., & Peigné, J. (2014). Agroecological practices for sustainable agriculture. A review. Agronomy for sustainable development. 34(1), 1-20. DOI 10.1007/s13593-013-0180-7.
Williams, A., Börjesson, G., & Hedlund, G. (2013). The effects of 55 years of different inorganic fertilizer regimes on soil properties and microbial community composition. Soil Biol. Biochem. 67, 41–46. https://doi.org/10.1016/j.soilbio.2013.08.008.
Wilson, D. B. (2011). Microbial diversity of cellulose hydrolysis. Current opinion in microbiology, 14(3), 259-263.
Zhang, K., Maltais-Landry, G., & Liao, H. L. (2021). How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biology and Biochemistry, 156, 108219.
Zhou, X., Yu, G., & Wu, F. (2011). Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. European Journal of Soil Biology, 47(5), 279-287.
Revista científica de la Asociación Argentina de la Ciencia del Suelo